8. Thermochemistry
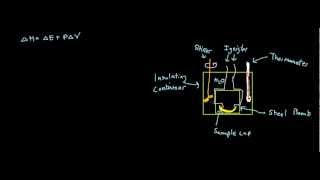
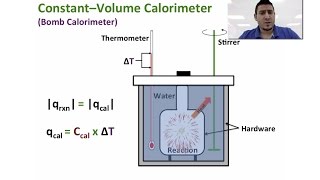
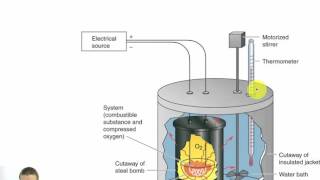
Constant-Volume Calorimetry

Get help from an AI Tutor
Ask a question to get started.
Problem 74
Textbook Question
Textbook QuestionMothballs are composed primarily of the hydrocarbon naphthalene (C10H8). When 1.025 g of naphthalene burns in a bomb calorimeter, the temperature rises from 24.25 °C to 32.33 °C. Find ΔErxn for the combustion of naphthalene. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.11 kJ/°C.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
3567
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos