11. Bonding & Molecular Structure
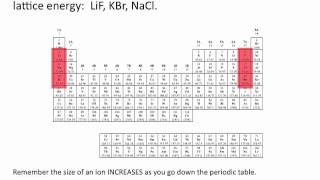
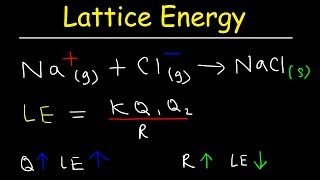
Lattice Energy
Problem 44
Textbook Question
Textbook QuestionRubidium iodide has a lattice energy of -617 kJ>mol, while potassium bromide has a lattice energy of -671 kJ>mol. Why is the lattice energy of potassium bromide more exothermic than the lattice energy of rubidium iodide?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1409
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos