15. Chemical Kinetics
Rate Law
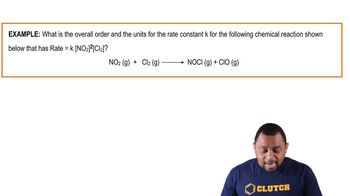
Problem 38c
Textbook Question
Textbook QuestionThis reaction is first order in N2O5: N2O5( g) ¡ NO3( g) + NO2( g) The rate constant for the reaction at a certain temperature is 0.053>s. b. What would the rate of the reaction be at the concentration indicated in part a if the reaction were second order? Zero order? (Assume the same numerical value for the rate constant with the appropriate units.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2722
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos