3. Chemical Reactions
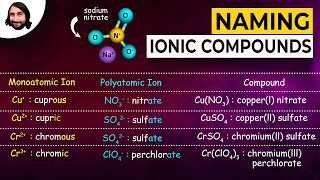
Naming Ionic Compounds
Problem 113b
Textbook Question
Textbook QuestionThe arsenic in a 1.22-g sample of a pesticide was converted to AsO43- by suitable chemical treatment. It was then titrated using Ag+ to form Ag3AsO4 as a precipitate. (b) Name Ag3AsO4 by analogy to the corresponding compound containing phosphorus in place of arsenic.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
743
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos