6. Chemical Quantities & Aqueous Reactions
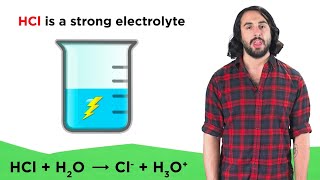
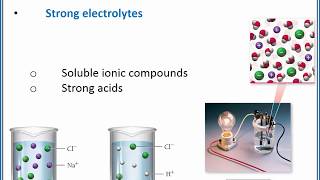
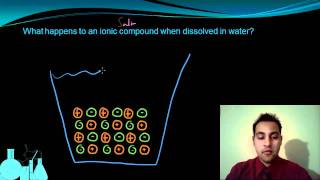
Electrolytes
Problem 62
Textbook Question
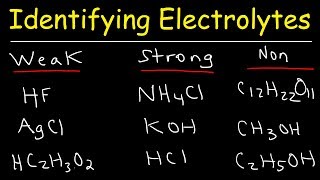
Textbook QuestionThe following aqueous solutions were tested with a light bulb conductivity apparatus, as shown in Figure 4.3. What result—dark, dim, or bright—do you expect from each? (a) 0.10 M potassium chloride (b) 0.10 M methanol (c) 0.10 M acetic acid

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1054
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos