13. Liquids, Solids & Intermolecular Forces
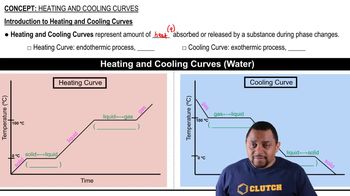
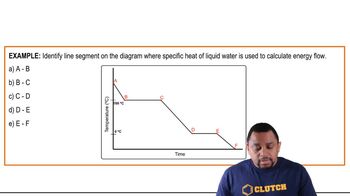
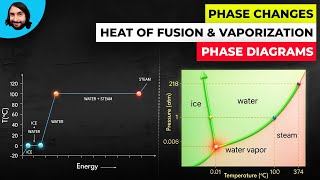
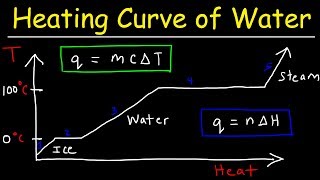
Heating and Cooling Curves
Problem 69
Textbook Question
Textbook QuestionAn 8.5-g ice cube is placed into 255 g of water. Calculate the temperature change in the water upon the complete melting of the ice. Assume that all of the energy required to melt the ice comes from the water.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
3314
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos