10. Periodic Properties of the Elements
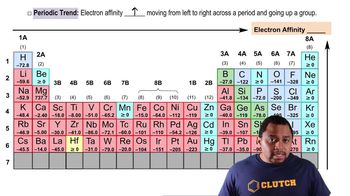
Periodic Trend: Electron Affinity

Get help from an AI Tutor
Ask a question to get started.
Problem 71
Textbook Question
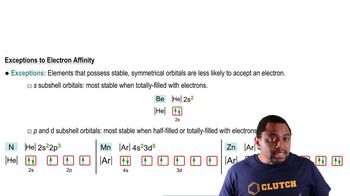
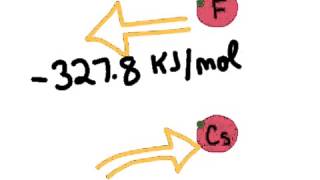
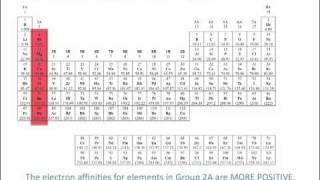
Textbook QuestionWhy does ionization energy increase regularly across the periodic table from group 1A to group 8A, whereas electron affinity increases irregularly from group 1A to group 7A and then falls dramatically for group 8A?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
306
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos