11. Bonding & Molecular Structure
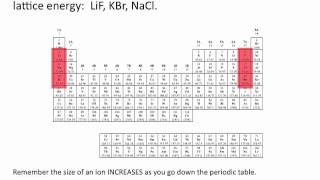
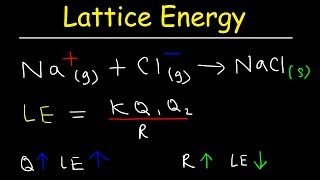
Lattice Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 80
Textbook Question
Textbook QuestionCalculate the energy change in kilojoules per mole when lithium atoms lose an electron to bromine atoms to form isolated Li+ and Br-ions. [The Ei for Li1g2 is 520 kJ/mol; the Eea for Br1g2 is -325 kJ/mol.] Will a lithium atom transfer an elec-tron to a bromine atom to form isolated Li+ 1g2 and Br-1g2 ions? Explain.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
318
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos