9. Quantum Mechanics
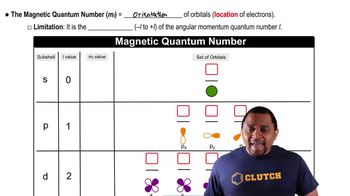
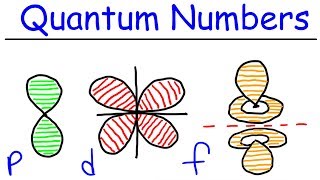
Quantum Numbers: Magnetic Quantum Number
Problem 62
Textbook Question
Textbook QuestionFor the table that follows, write which orbital goes with the quantum numbers. Don't worry about x, y, z subscripts. If the quantum numbers are not allowed, write 'not allowed.' n l ml Orbital 2 1 -1 2p (example) 1 0 0 3 -3 2 3 2 -2 2 0 -1 0 0 0 4 2 1 5 3 0

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1028
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos