15. Chemical Kinetics
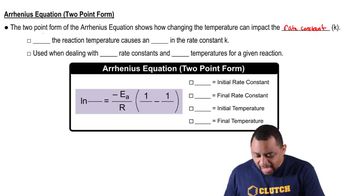
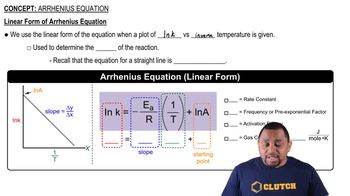
Arrhenius Equation
Problem 112a
Textbook Question
Textbook QuestionEthyl chloride vapor decomposes by the first-order reaction: C2H5Cl¡C2H4 + HCl The activation energy is 249 kJ>mol, and the frequency factor is 1.6 * 1014 s - 1. Find the value of the rate constant at 710 K.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
184
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos