10. Periodic Properties of the Elements
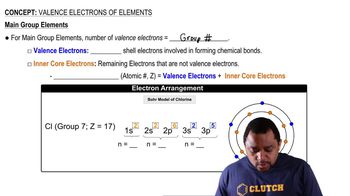
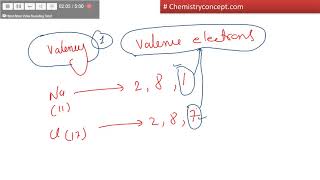
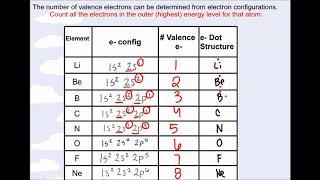
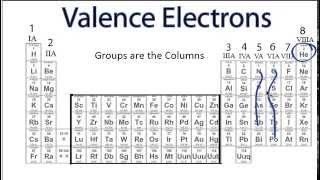
Valence Electrons of Elements
Problem 12c
Textbook Question
Textbook Question(c) Ti and Hf behave as though they possess the same number of valence electrons. Which of the subshells in the electron configuration of Hf behave as valence orbitals? Which behave as core orbitals?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
682
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos