15. Chemical Kinetics
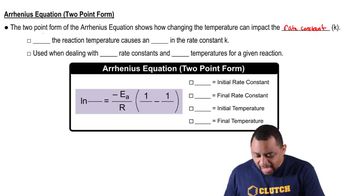
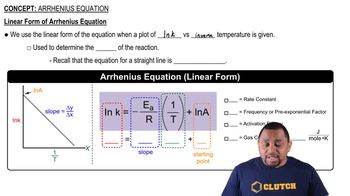
Arrhenius Equation
Problem 63
Textbook Question
Textbook QuestionThe rate constant (k) for a reaction was measured as a function of temperature. A plot of ln k versus 1>T (in K) is linear and has a slope of -7445 K. Calculate the activation energy for the reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
6580
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos