Multiple Choice
Given the structure , how would you classify this amine?
39
views
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: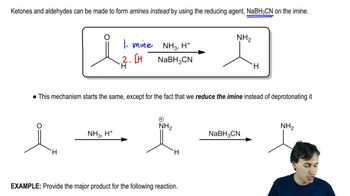

 6:50m
6:50mMaster Naming Primary Amines with a bite sized video explanation from Johnny
Start learning