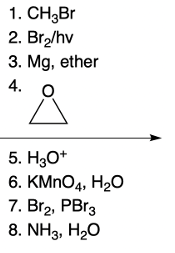
Amino acid synthesis can be effectively achieved through the Hell, Bohard, and Zelinsky (Hvz) method, which involves a two-step process. The first step focuses on the Hvz reaction, where a bromine atom replaces the alpha hydrogen atom of a carboxylic acid. In this reaction, the alpha carbon is crucial, as it is the carbon adjacent to the carboxyl group. The reaction utilizes bromine (Br2) in the presence of a catalyst, phosphorus tribromide (PBr3), to facilitate the displacement of the alpha hydrogen, resulting in the formation of alpha-bromo carboxylic acid.
In the second step, known as the amination step, ammonia (NH3) attacks the alpha carbon through a nucleophilic substitution reaction (SN2). This process displaces the bromine atom, leading to the formation of an alpha amino acid, characterized by the presence of the amino group (NH2) at the alpha position. This method highlights a systematic approach to synthesizing amino acids, integrating both the Hvz reaction and subsequent amination to achieve the desired product.