For the following values of ∆H° , ∆S°, and T, tell whether the process would be favored.
(c) ∆H° = -21.3 kcal/mol ; AS° = -51 cal/mol•K ; T = 373 K
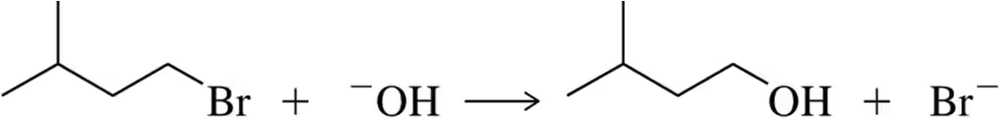
![Table displaying concentrations of [RBr] and [HO-] with corresponding reaction rates for control and trials.](https://static.studychannel.pearsonprd.tech/courses/organic-chemistry/thumbnails/1c9e5ad1-b4d8-44b4-9933-02c7b65962db)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 5:02m
5:02mMaster Breaking down the different terms of the Gibbs Free Energy equation. with a bite sized video explanation from Johnny
Start learning