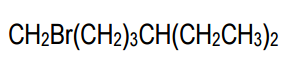Understanding condensed structures is essential for representing organic molecules efficiently, especially when working with text-based formats. The condensed structure method emerged as a practical solution for chemists who needed to describe molecular connectivity without the aid of graphical software, relying instead on typewriters and simple text notation.
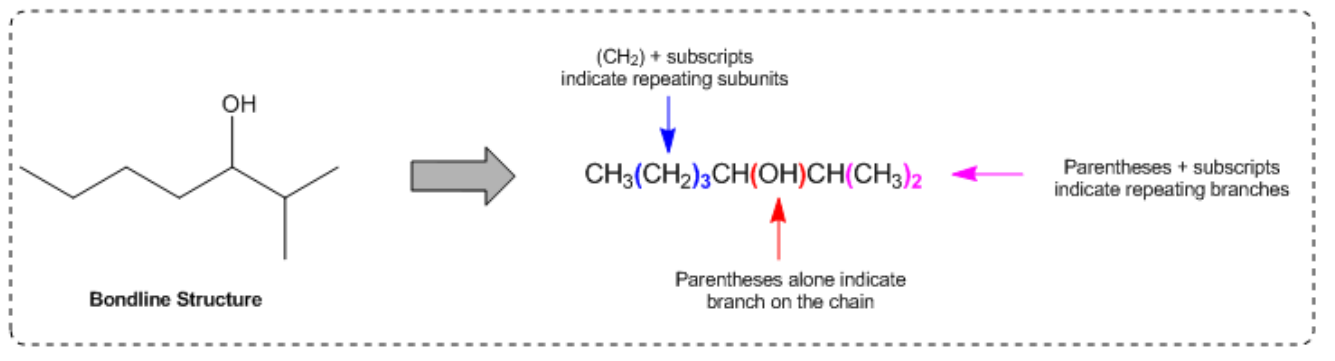
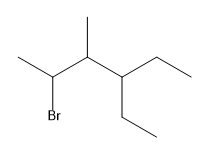
In condensed structures, the connectivity of atoms is conveyed through a linear text format. For instance, a full condensed structure might represent a molecule like CH3CH2CH2OH in a single line, making it easier to visualize the connections. A key feature of this notation is the use of parentheses to indicate repeating units or branches. For example, CH2(CH2)3 signifies a linear chain of three CH2 groups, rather than three separate CH2 units. This notation is particularly useful for long carbon chains, allowing chemists to avoid writing out extensive sequences.
Another important aspect of condensed structures is the representation of branches. When a carbon atom has fewer than four bonds, parentheses can indicate additional groups attached to it. For example, if a carbon is connected to an OH group and an H atom, it may be represented as (OH) or simply as part of a longer chain, depending on the context. Understanding how to interpret these structures is crucial, as sometimes parentheses may be omitted, requiring the chemist to deduce the branching based on the connectivity rules of carbon.
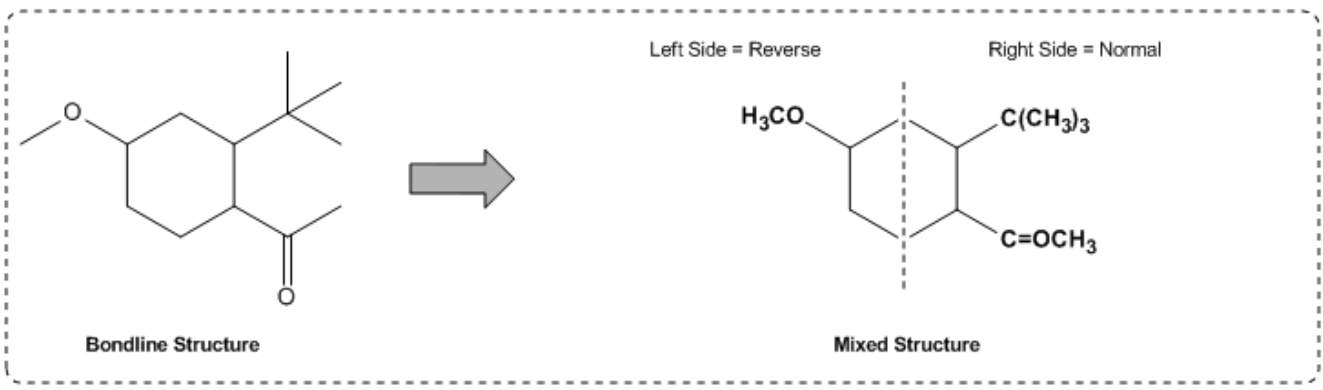
Additionally, when multiple groups are attached to a single carbon, such as CH3(CH3)2, it indicates that both CH3 groups are branching off the same carbon atom. This contrasts with linear representations, where repeating units are indicated by CH2 in parentheses. The ability to visualize and convert between condensed and bond-line structures is vital, as these formats are often used interchangeably in academic settings.
Ultimately, mastering condensed structures involves recognizing how to ensure that each carbon atom maintains four bonds, a fundamental principle in organic chemistry. This skill not only aids in understanding molecular structures but also enhances problem-solving capabilities when interpreting complex organic compounds.